Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Murakami, Takashi*; Isobe, Hiroshi; Sato, Tsutomu; Onuki, Toshihiko
Clays and Clay Minerals, 44(2), p.244 - 256, 1996/00
Times Cited Count:55 Percentile:84.43(Chemistry, Physical)no abstracts in English
*
PNC TJ1211 95-007, 117 Pages, 1995/02
1. Survey on leaching rate of pyrite, chlorite, epidote and siderite. (1) Pyrite Reaction rate (K) is depended on dissolve O concentration in Lin's literature. 1 - (1-x)
concentration in Lin's literature. 1 - (1-x) 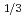 = k [O
= k [O ]
] 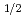 ・t (x: Mole number of dissolved FeS
・t (x: Mole number of dissolved FeS , [O
, [O ]: Dissolve O
]: Dissolve O concentration. T:Time) Reaction rate is changed by temperature. k = 2.2
concentration. T:Time) Reaction rate is changed by temperature. k = 2.2  10
10 exp(-9140/T) (2) chlorite Reaction rate is measured 2.7
exp(-9140/T) (2) chlorite Reaction rate is measured 2.7  6.7
6.7  10
10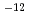 mol/m
mol/m /s(pH3
/s(pH3  4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
 10
10 mol/cm
mol/cm /s in pH1
/s in pH1  11 by Rose. (4) Siderite Reaction rate is measured 9.93
11 by Rose. (4) Siderite Reaction rate is measured 9.93  10
10 mol/m
mol/m /s in O
/s in O -free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
-free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
 10
10 (cm
(cm ・mol
・mol ) 1/2・h
) 1/2・h by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
 10
10 by Ross's rate equation.
by Ross's rate equation. 
 = kt [
= kt [  : disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.
: disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.
Onuki, Toshihiko; Murakami, Takashi; Sekine, Keiichi; Yanase, Nobuyuki; Isobe, Hiroshi; Kobayashi, Yoshii
Materials Research Society Symposium Proceedings, Vol. 176, p.607 - 614, 1990/00
no abstracts in English